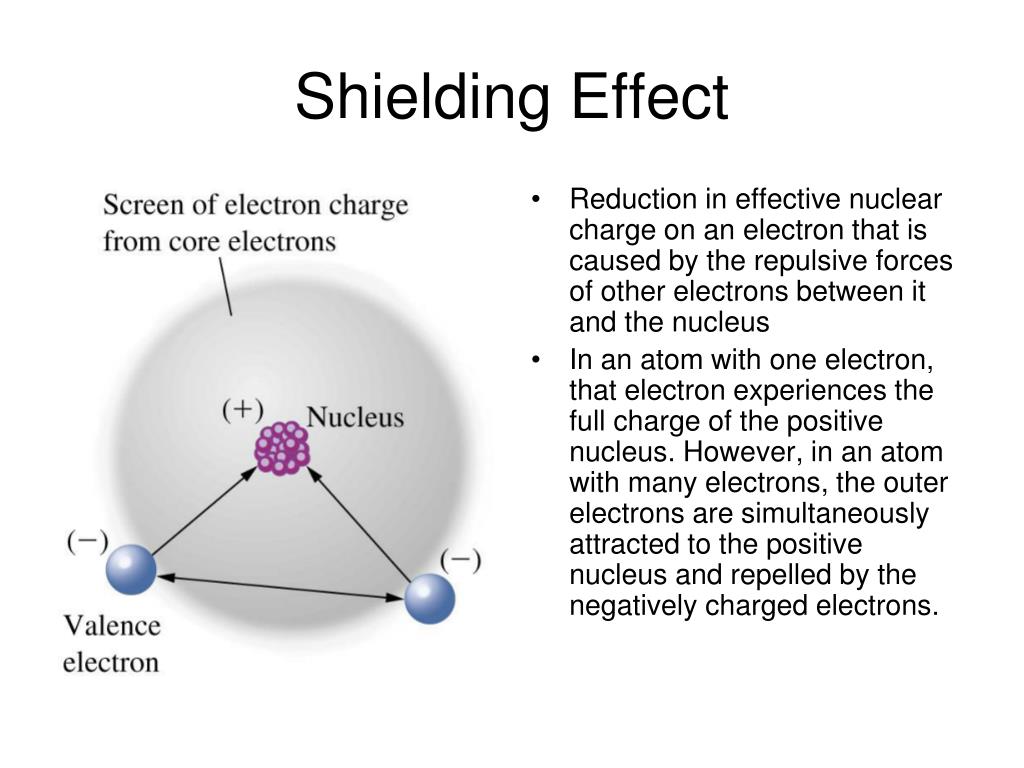
Why Is Electron Shielding Not A Factor When You . the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. however, due to the repulsive forces from the inner electrons, the outer electrons experience a reduced attractive force. shielding is the reduction of true nuclear charge (z) to the effective nuclear charge (z*) by other electrons in a. this is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. In other words, the inner.
from www.slideserve.com
shielding is the reduction of true nuclear charge (z) to the effective nuclear charge (z*) by other electrons in a. In other words, the inner. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. this is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. however, due to the repulsive forces from the inner electrons, the outer electrons experience a reduced attractive force.
PPT Periodic Trends PowerPoint Presentation, free download ID6734591
Why Is Electron Shielding Not A Factor When You the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. however, due to the repulsive forces from the inner electrons, the outer electrons experience a reduced attractive force. this is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. In other words, the inner. shielding is the reduction of true nuclear charge (z) to the effective nuclear charge (z*) by other electrons in a.
From exoeftfzx.blob.core.windows.net
What Is Shielding Chem at Victor Riggs blog Why Is Electron Shielding Not A Factor When You however, due to the repulsive forces from the inner electrons, the outer electrons experience a reduced attractive force. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. In other words, the inner. The shielding effect describes the balance between the pull of the protons. Why Is Electron Shielding Not A Factor When You.
From www.youtube.com
why shielding effect of electrons makes cation formation easy ?9th Why Is Electron Shielding Not A Factor When You In other words, the inner. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. however, due to the repulsive forces from the inner electrons, the outer electrons experience a reduced attractive force. this is because of shielding, or simply the electrons closest to. Why Is Electron Shielding Not A Factor When You.
From slideplayer.com
The Periodic Table. ppt download Why Is Electron Shielding Not A Factor When You In other words, the inner. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. shielding is the reduction of true nuclear charge (z) to the effective nuclear charge (z*) by other electrons in a. the concept of electron shielding, in which intervening electrons act to reduce. Why Is Electron Shielding Not A Factor When You.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation ID3646738 Why Is Electron Shielding Not A Factor When You the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. In other words, the inner. this is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. however, due to the repulsive forces. Why Is Electron Shielding Not A Factor When You.
From ceyzfvbm.blob.core.windows.net
V) What Is Meant By Shielding Of Electrons In An Atom at Christina Why Is Electron Shielding Not A Factor When You this is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The shielding effect describes the balance between the pull of the protons. Why Is Electron Shielding Not A Factor When You.
From ar.inspiredpencil.com
Electron Shielding Why Is Electron Shielding Not A Factor When You In other words, the inner. this is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. however, due to the repulsive forces from the inner. Why Is Electron Shielding Not A Factor When You.
From socratic.org
How are shielding effect and atomic radius related? Socratic Why Is Electron Shielding Not A Factor When You however, due to the repulsive forces from the inner electrons, the outer electrons experience a reduced attractive force. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. shielding is the reduction of true nuclear charge (z) to the effective nuclear charge (z*) by other electrons in. Why Is Electron Shielding Not A Factor When You.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2834450 Why Is Electron Shielding Not A Factor When You In other words, the inner. this is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. however, due to the repulsive forces from the inner electrons, the outer electrons experience a reduced attractive force. The shielding effect describes the balance between the pull of the protons. Why Is Electron Shielding Not A Factor When You.
From cemizhaf.blob.core.windows.net
Why Is Electron Shielding Not A Factor When You Examine A Trend Across Why Is Electron Shielding Not A Factor When You however, due to the repulsive forces from the inner electrons, the outer electrons experience a reduced attractive force. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The shielding effect describes the balance between the pull of the protons on valence electrons and the. Why Is Electron Shielding Not A Factor When You.
From www.youtube.com
Shielding effect of Electroninner Electrons on outer Electronin the Why Is Electron Shielding Not A Factor When You shielding is the reduction of true nuclear charge (z) to the effective nuclear charge (z*) by other electrons in a. this is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. In other words, the inner. the concept of electron shielding, in which intervening electrons. Why Is Electron Shielding Not A Factor When You.
From cemizhaf.blob.core.windows.net
Why Is Electron Shielding Not A Factor When You Examine A Trend Across Why Is Electron Shielding Not A Factor When You this is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The shielding effect describes the balance between the pull of the protons. Why Is Electron Shielding Not A Factor When You.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e Why Is Electron Shielding Not A Factor When You The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. this is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. In other words, the inner. shielding is the reduction of true nuclear charge (z). Why Is Electron Shielding Not A Factor When You.
From slidetodoc.com
Factors that affect electron energies Shielding provided by Why Is Electron Shielding Not A Factor When You this is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. In other words, the inner. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. shielding is the reduction of true nuclear charge (z). Why Is Electron Shielding Not A Factor When You.
From www.slideserve.com
PPT Chapter 6 Periodic Table PowerPoint Presentation, free download Why Is Electron Shielding Not A Factor When You this is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. shielding is the reduction of true nuclear charge (z) to the effective nuclear charge. Why Is Electron Shielding Not A Factor When You.
From slideplayer.com
Periodic Trends. ppt download Why Is Electron Shielding Not A Factor When You In other words, the inner. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. shielding is the reduction of true nuclear charge (z). Why Is Electron Shielding Not A Factor When You.
From cemizhaf.blob.core.windows.net
Why Is Electron Shielding Not A Factor When You Examine A Trend Across Why Is Electron Shielding Not A Factor When You this is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. shielding is the reduction of true nuclear charge (z) to the. Why Is Electron Shielding Not A Factor When You.
From slideplayer.com
Effective Nuclear Charge, Shielding, and Trends on the Periodic Table Why Is Electron Shielding Not A Factor When You however, due to the repulsive forces from the inner electrons, the outer electrons experience a reduced attractive force. this is because of shielding, or simply the electrons closest to the nucleus decrease the amount of nuclear charge affecting the outer electrons. shielding is the reduction of true nuclear charge (z) to the effective nuclear charge (z*) by. Why Is Electron Shielding Not A Factor When You.
From www.uochemists.com
Electron Shielding UO Chemists Why Is Electron Shielding Not A Factor When You however, due to the repulsive forces from the inner electrons, the outer electrons experience a reduced attractive force. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. shielding is the reduction of true nuclear charge (z) to the effective nuclear charge (z*) by. Why Is Electron Shielding Not A Factor When You.